![]()
Urgent Message: Urgent care teams can take practical steps to safeguard quality for Clinical Laboratory Improvement Amendments (CLIA)-waived testing by following detailed Instructions for Use information.
Key Words: Clinical Laboratory Improvement Amendments; CLIA-Waived Testing; Certificate of Waiver
Laboratory tests commonly used in urgent care are only reliable when their Instructions for Use (IFU)—including any limitations—are followed. For urgent care leaders, this isn’t just about compliance with federal regulations. Patient outcomes, safety, and even a site’s Clinical Laboratory Improvement Amendments (CLIA)-waived status depend on it. Because IFU limitations are often a “blind spot” in urgent care, this article also explores why they matter, common pitfalls, and practical steps urgent care teams can take to safeguard quality.
Waived Doesn’t Mean Worry-Free
On a busy weekday afternoon, an urgent care provider may order a CLIA-waived test for a patient with sore throat, cough, vaginitis, or urinary complaints, as just a few examples. Results may come back within minutes, shaping treatment decisions, yet few pause to consider the fine print in the test’s IFU, which may affect the accuracy of the test result.
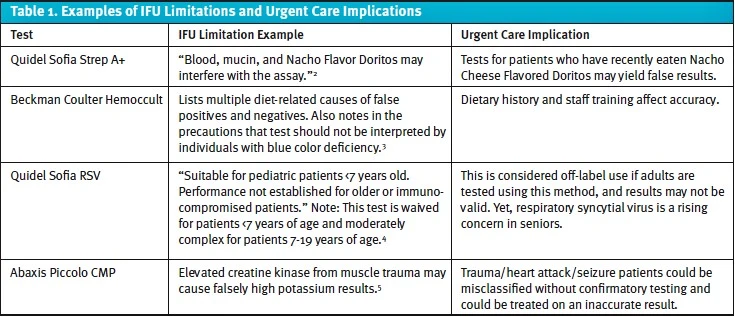
IFUs do more than explain how to run the test. They also include limitations that can lead to significant consequences if ignored, such as incorrect results, missed diagnoses, inappropriate treatment, and regulatory non-compliance for the medical director. A framework for embedding IFU limits into workflow and purchasing decisions, however, can help reduce callbacks, re-collections, and regulatory risk.
CLIA Waived Testing
Under CLIA, waived tests are defined as test with a low risk of incorrect results. To perform them, a facility must hold a valid Certificate of Waiver and follow the manufacturer’s IFU precisely, including the limitations section. Direct oversight for CLIA-waived tests is typically light with inspections occurring randomly, in response to complaints, or when the Centers for Medicare & Medicaid Services—which administers the overall program, issues certificates, and enforces regulations for laboratories—has reason to believe testing poses risk.1 Even so, compliance is everyone’s responsibility.
Many operators assume their waived certificate is sufficient to run waived tests, however, following IFUs in everyday practice is equally critical. Failure to follow IFUs can reclassify a test as high complexity, requiring added oversight, training, and inspections. Additionally, a medical director may be held personally responsible for consequences such as incorrect results, patient harm, or loss of the site’s waived status when staff doesn’t follow IFUs.
What IFU Limitations Look Like
Limitations often describe conditions that may lead to inaccurate or uninterpretable results. Many seem intuitive, such as inadequate specimen collection, but others can be surprising. Table 1 provides a few examples of the many limitations that can affect urgent care interpretation.
Why Limitations Matter in Urgent Care
Urgent care centers tend to operate with small teams, often including medical assistants with limited lab training. High turnover and heavy patient volume create a situation in which IFUs may only be reviewed for the initial setup and not reinforced with staff regularly. That creates risk across multiple fronts.
- Patient safety: Misinterpreted results can lead to inappropriate treatment or missed diagnoses.
- Public health: For communicable diseases like sexually transmitted infections (STIs) or COVID, false negatives mean ongoing transmission.
- Regulatory compliance: Deviating from IFUs puts the urgent care center’s non-waived classification at risk and may result in penalties.
Hypothetical Case Example
Consider a hypothetical presentation of a 22-year-old patient with dysuria and vaginal discharge. A waived nucleic acid amplification test (NAAT) for chlamydia and gonorrhea might be ordered. The test’s IFU notes that certain, non-approved swab types can cause false results, but the staff member collecting the specimen may not be aware of the limitation and use a non-approved swab for collection. The patient is soon reassured of a negative result and is sent home without treatment.
Days later, the patient is diagnosed at a different site of care with pelvic inflammatory disease, and it is believed the non-compliant swab used in the NAAT may have caused a false negative at the outset, likely causing unnecessary disease progression that may have been avoided with earlier diagnosis.
Common Categories of Limitations
While specifics vary, most waived tests include limitations in a few broad categories. Urgent care teams must be aware of these pitfalls to interpret results responsibly.
- Specimen integrity: Adequate collection, storage, and transport are essential.
- Population restrictions: Many tests are validated only for certain age groups or patient types.
- Cross-reactivity: Interference from other organisms, foods, or medications may cause false positives or negatives.
- Visual interpretation: Colorblindness in the interpreter or subjective qualities of color changes can affect results.
- Sample volume and contamination: Insufficient material or cross-contamination can skew polymerase chain reaction and other assays.
Making It Actionable
One way to operationalize IFU limitations is by embedding them into a simple pretest questionnaire that staff can complete quickly at the point of care. By standardizing these checks, urgent care centers reduce variability and improve compliance.
Sample Pretest Screening Questions
- Has the patient eaten within the past 30 minutes?
- What is the patient’s age? Is the test validated for this age group?
- Does the patient have other conditions (eg, muscle trauma; immunocompromised) that could interfere with results?
- Was the specimen collected, labeled, and transported according to instructions?
- Does the patient’s clinical presentation align with test results?
Comparing STI Waived Assays
STI testing is an important area for urgent care with significant impact on both individual patients and public health. Different waived assays vary in their IFU limitations, and these differences should be considered when bringing in new testing. The examples in Table 2 are illustrative, not exhaustive. Always verify IFUs for complete details.
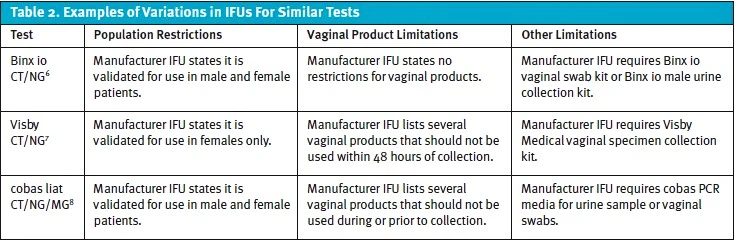
Limitations vary widely—some platforms have multiple limitations—and should be reviewed often for potential impact. Operational leaders should assess IFU limitations against their site’s workflow and patient mix, for example.
Steps for Urgent Care Leaders
Urgent care directors and administrators can take immediate steps to make IFU compliance part of daily workflow. Consider starting with a simple checklist approach.
- Run a quick audit of your top 5 waived tests and document their IFUs, including the limitations.
- Create a 6–8 item pretest checklist and laminate copies for triage and other staff stations.
- Embed IFU guardrails into your electronic health record order sets to reinforce correct use at the point of care.
- Train staff on why IFU limitations matter for patient outcomes and compliance.
- Evaluate new platforms by including IFU limitations as a scored criterion during selection.
- Implement ongoing quality checks with extra attention to high-impact tests.
By operationalizing IFUs in this way, urgent care leaders can reduce risk and improve consistency of testing across their teams.
Conclusion
Urgent care thrives on speed and efficiency, but accuracy is equally critical. CLIA-waived status comes with ongoing responsibility beyond obtaining the Certificate of Waiver. By bringing the IFU limitations into daily practice through staff education, checklists, and thoughtful test selection, urgent care leaders can protect patients, maintain compliance, and strengthen public health outcomes.
The fine print matters. Now is the time to make it part of your frontline workflow.
References
- Clinical Laboratory Improvement Amendments (CLIA) Test Complexities. Centers for Disease Control and Prevention. https://www.cdc.gov/clia/php/test-complexities/index.html. Accessed September 2, 2025.
- Quidel Sofia Strep A+ FIA package insert. REF 20274 – Sofia Strep A+ FIA – 25 Test. 12-2014 https://static.webareacontrol.com/CommonFile/instructions-for-use-1678096459246.pdf Accessed September 2, 2025.
- Beckman Coulter Hemoccult Product Instructions. 462478EC.pdf. March 2015.
- Quidel Sofia RSV IFU. CL20260_EN. 2017.
- Piccolo Comprehensive Metabolic Panel IFU. PN: 400-7139-1. February 2025.
- Binx io Instructions for Use. 1.002.101 MOB-ART 0700 v4.0. May 2022.
- Visby Medical CT/NG IFU. PS-300648 Rev B. September 2023.
- cobas liat CT/NG/MG Instructions for Use. 10147555001-01EN. Rev 1.0. January 2025.
Download the article PDF: Why Ifu Limitations Matter In Urgent Care Lab Testing
 480-245-6400
480-245-6400